学術論文および総説
2020
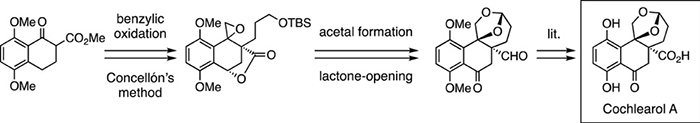
- Formal synthesis of cochlearol A, a meroterpenoid with renoprotective activity
Naruse, K.; Katsuta, R.; Yajima, A.; Nukada, T.; Watanabe, H.; Ishigami, K.
Tetrahedron Lett. 61, 151845 (2020).
doi:10.1016/j.tetlet.2020.151845
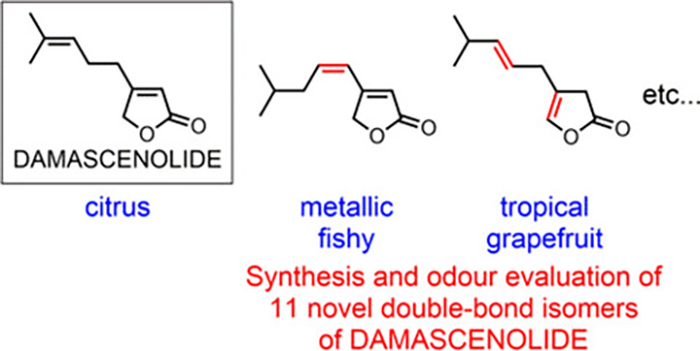
- Synthesis and odour evaluation of double-bond isomers of DAMASCENOLIDE, 4-(4-methylpent-3-en-1-yl)-2(5H)-furanone, which has a citrus-like odour
Miyazawa, Y.; Ohashi, T.; Kawaguchi, K.; Tanaka, N.; Katsuta, R.; Yajima, A.; Nukada, T.; Ishigami, K.
Flavor Fragra. J. 35, 341-349 (2020).
doi:10.1002/ffj.3568
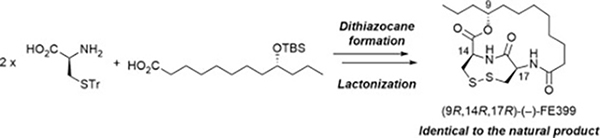
- Synthesis and stereochemistry of (−)-FE399
Katsuta, R.; Masada, N.; Kimura, K.; Yajima, A.; Ishigami, K.; Nukada, T.
Tetrahedron Lett. 61, 151783 (2020).
doi:10.1016/j.tetlet.2020.151783
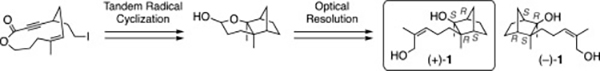
- Synthesis of anti-Helicobacter pylori sesquiterpene employing tandem radical cyclization, and determination of the absolute configuration of the natural product
Ishigami, K.; Kadowaki, K.; Yamada, S.; Aiba, S.; Kawasaki, T.; Katsuta, R.; Yajima, A.; Nukada, T.; Takikawa, H.; Watanabe, H.
Tetrahedron 76, 130834 (2020).
doi:10.1016/j.tet.2019.130834
2019
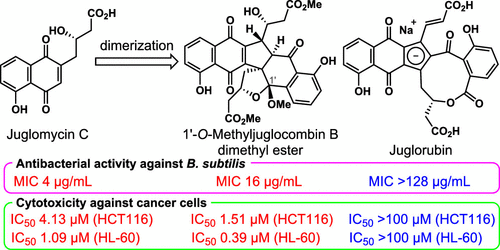
- Synthetic and biological studies of juglorubin and related naphthoquinones
Kamo, S.; Saito, T.; Kusakabe, Y.; Tomoshige, S.; Uchiyama, M.; Tsubaki, K.; Kuramochi, K.
J. Org. Chem. 84, 13967-13974 (2019).
doi:10.1021/acs.joc.9b02119
2018
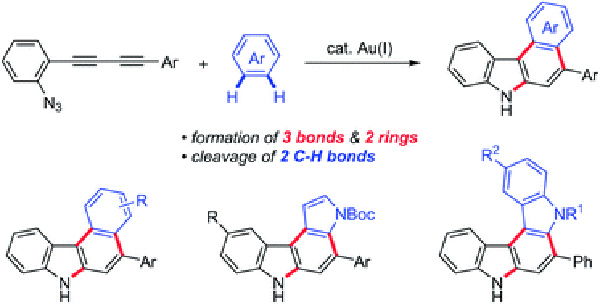
- Direct synthesis of aryl-annulated [c]carbazoles by gold(I)-catalysed cascade reaction of azide-diynes and arenes
Kawada, Y.; Ohmura, S.; Kobayashi, M.; Nojo, W.; Kondo, M.; Matsuda, Y.; Matsuoka, J.; Inuki, S.; Oishi, S.; Wang, C.; Saito, T.; Uchiyama, M.; Suzuki, T.; Ohno, H.
Chem. Sci. 9, 8416-8425 (2018).
doi:10.1039/C8SC03525C
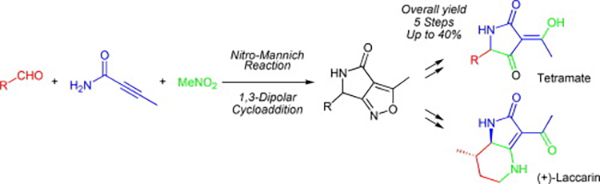
- Nitro-Mannich reaction and intramolecular 1,3-dipolar cycloaddition route to acylpyrrolidinones: Synthesis of a tetramic acid and (+)-laccarin
Katsuta, R.; Ichijo, H.; Oouchi, G.; Yajima, A.; Ishigami, K.; Nukada, T.
Tetrahedron Lett. 59, 2352-2355 (2018).
doi:10.1016/j.tetlet.2018.05.014
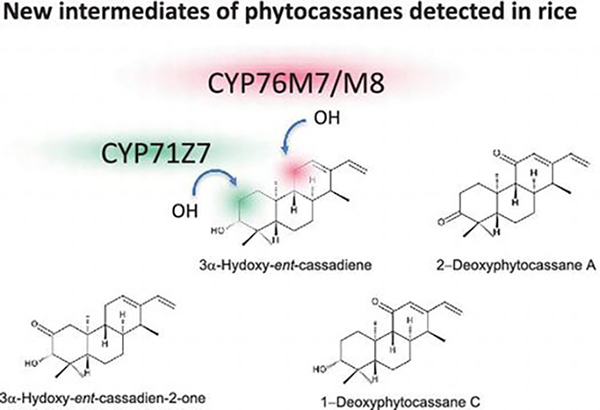
- In planta functions of cytochrome P450 monooxygenase genes in the phytocassane biosynthetic gene cluster on rice chromosome 2
Ye, Z.; Yamazaki, K.; Minoda, H.; Miyamoto, K.; Miyazaki, S.; Kawaide, H.; Yajima, A.; Nojiri, H.; Yamane, H.; Okada, K.
Biosci. Biotechnol. Biochem. 82, 1021-1030 (2018).
doi:10.1080/09168451.2017.1398067
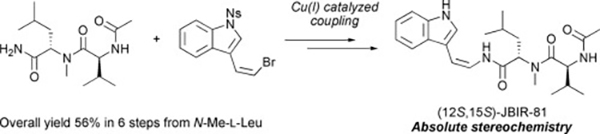
- Synthesis and stereochemistry of JBIR-81, a peptide enamide derived from aspergilli
Katsuta, R.; Toyoda, M.; Yajima, A.; Ishigami, K.; Nukada, T.
Tetrahedron Lett. 59, 1010-1013 (2018).
doi:10.1016/j.tetlet.2018.01.080
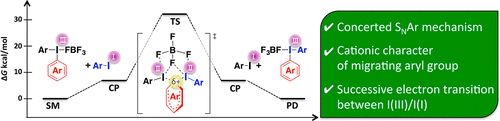
- Mechanistic study on aryl exchange reaction of diaryl-λ3-iodane with aryl iodide
Masumoto, Y.; Miyamoto, K.; Iuchi, T.; Ochiai, M.; Hirano, K.; Saito, T.; Wang, C.; Uchiyama, M.
J. Org. Chem. 83, 289-295 (2018).
doi:10.1021/acs.joc.7b02701
- (Review Article) 元素の特性を活用した反応開発と天然物合成への応用
斉藤竜男
Yakugaku Zasshi 138, 1335-1344 (2018).
doi:10.1248/yakushi.18-00126
2017
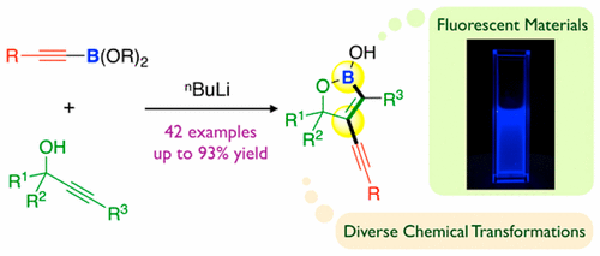
- Transition metal-free trans-selective alkynylboration of alkynes
Nogami, M.; Hirano, K.; Kanai, M.; Wang, C.; Saito, T.; Miyamoto, K.; Muranaka, A.; Uchiyama, M.
J. Am. Chem. Soc. 139, 12358-12361 (2017).
doi:10.1021/jacs.7b06212
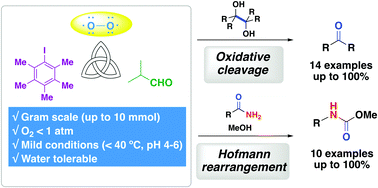
- Iodoarene-catalyzed oxidative transformations using molecular oxygen
Miyamoto, K.; Yamashita, J.; Narita, S.; Hirano, K.; Saito, T.; Wang, C.; Ochiai, M.; Uchiyama, M.
Chem. Commun. 53, 9781-9784 (2017).
doi:10.1039/C7CC05160C
- Convergent synthesis of the ent-ZA’B’C’D’-ring system of maitotoxin
Saito, T.; Morita, M.; Koshino, H.; Sodeoka, M.; Nakata, T.
Org. Lett. 19, 3203-3206 (2017).
doi:10.1021/acs.orglett.7b01301
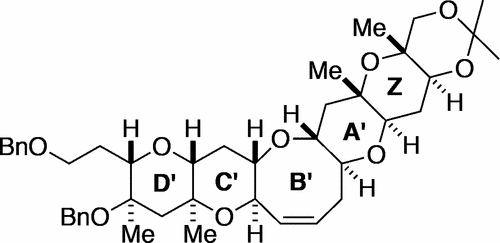
- Revisitation of organoaluminum reagents affords a versatile protocol for C–X (X = N, O, F) bond-cleavage cross-coupling: A systematic study
Ogawa, H.; Yang, Z.-K.; Minami, H.; Kojima, K.; Saito, T.; Wang, C.; Uchiyama, M.
ACS Catal. 7, 3988-3994 (2017).
doi:10.1021/acscatal.7b01058
- Synthesis and stereochemistry of decarestrictines H and J
Katsuta, R.; Masada, N.; Shimodaira, Y.; Ueda, S.; Yajima, A.; Nukada, T.
Tetrahedron 73, 1733-1739 (2017).
doi:10.1016/j.tet.2017.02.023
2016
- Cross‐Coupling of Organolithium with Ethers or Aryl Ammonium Salts by C−O or C−N Bond Cleavage
Yang, Z.-K.; Wang, D.-Y.; Ogawa, H.; Minami, H.; Ozaki, T.; Saito, T.; Miyamoto, K.; Wang, C.; Uchiyama, M.
Chem. Eur. J. 22, 15693-15699 (2016).
doi:10.1002/chem.201603436
- Rhodium-catalyzed (perfluoroalkyl)olefination of acetanilides leading to perfluoroalkylated aromatics
Harada, K.; Tezuka, N.; Hirano, K.; Miyamoto, K.; Saito, T.; Uchiyama, M.
Chem. Pharm. Bull. 64, 1442-1444 (2016).
doi:10.1248/cpb.c16-00588
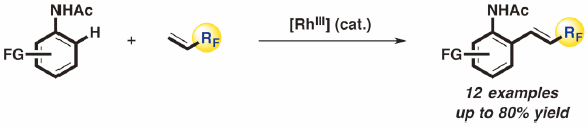
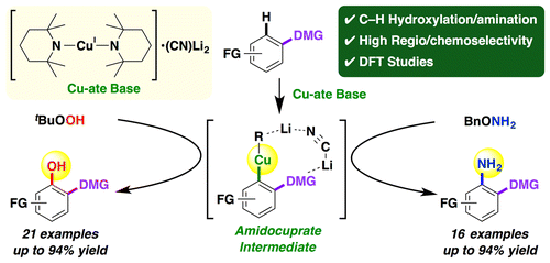
- Direct hydroxylation and amination of arenes via deprotonative cupration
Tezuka, N.; Shimojo, K.; Hirano, K.; Komagawa, S.; Yoshida, K.; Wang, C.; Miyamoto, K.; Saito, T.; Takita, R.; Uchiyama, M.
J. Am. Chem. Soc. 138, 9166-9171 (2016).
doi:10.1021/jacs.6b03855
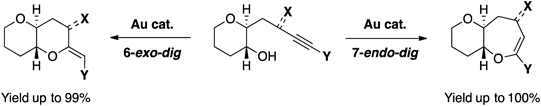
- Gold-catalyzed cyclization of alkyne alcohols: regioselective construction of functionalized 6,6- and 6,7-bicyclic ethers
Kubota, M.; Saito, T.; Miyamoto, K.; Hirano, K.; Wang, C.; Uchiyama, M.
Chem. Pharm. Bull. 64, 845-855 (2016).
doi:10.1248/cpb.c16-00204
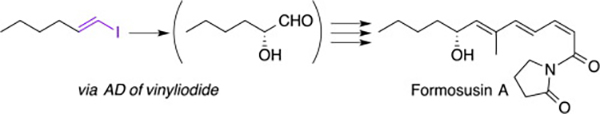
- Synthesis and absolute configuration of formosusin A, a specific inhibitor of mammalian DNA polymerase β
Yajima, A.; Iizuka, Y.; Katsuta, R.; Nukada, T.
Tetrahedron Lett. 57, 2012-2015 (2016).
doi:10.1016/j.tetlet.2016.03.094
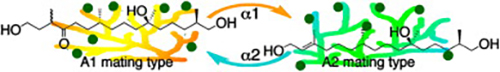
- The Phytophthora mating hormone α2 is an antagonist of the counter hormone α1
Zhang, L.; Yajima, A.; Ojika, M.
Biosci. Biotechnol. Biochem. 80, 1062-1065 (2016).
doi:10.1080/09168451.2016.1146071
- Allylic borylation of tertiary allylic alcohols: A divergent and straightforward access to allylic boronates
Harada, K.; Nogami, M.; Hirano, K.; Kurauchi, D.; Kato, H.; Miyamoto, K.; Saito, T.; Uchiyama, M.
Org. Chem. Front. 3, 565-569 (2016).
doi:10.1039/C6QO00009F

2015
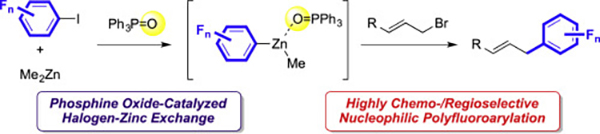
- Dialkylzinc-mediated allylic polyfluoroarylation reaction
Kurauchi, D.; Hirano, K.; Kato, H.; Saito, T.; Miyamoto, K.; Uchiyama, M.
Tetrahedron 71, 5849-5857 (2015).
doi:10.1016/j.tet.2015.05.107
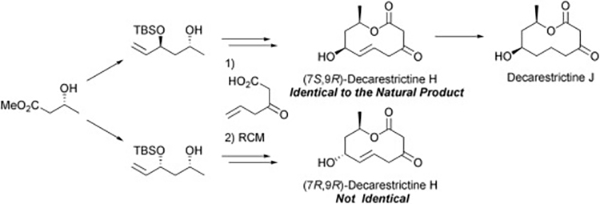
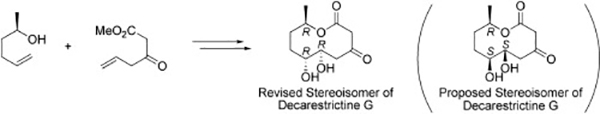
- Stereoselective synthesis of (-)-decarestrictine G
Katsuta, R.; Fujikawa, S.; Yajima, A.; Nukada, T.
Tetrahedron 71, 3428-3432 (2015).
doi:10.1016/j.tet.2015.03.082
- Direct construction of fused indoles by gold-catalyzed cascade cyclization of conjugated diynes
Naoe, S.; Saito, T.; Uchiyama, M.; Oishi, S.; Fujii, N.; Ohno, H.
Org. Lett. 17, 1774-1777 (2015).
doi:10.1021/acs.orglett.5b00550 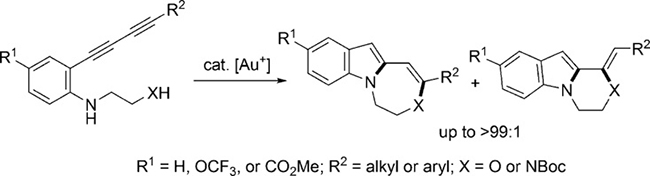
<Very Important Paper>
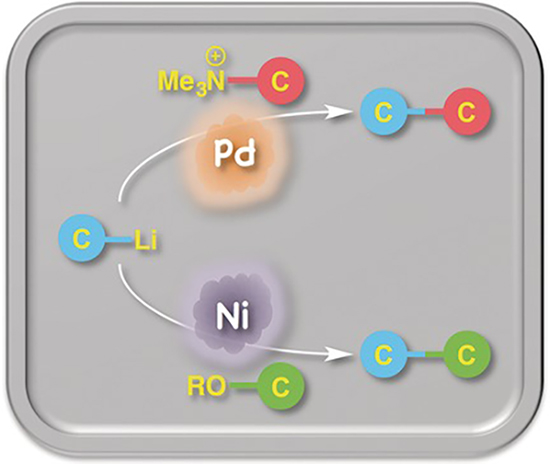
- Organoaluminum-mediated direct cross-coupling reaction
Minami, H.; Saito, T.; Wang, C.; Uchiyama, M.
Angew. Chem. Int. Ed. 54, 4665-4668 (2015).
doi:10.1002/anie.201412249

2014
- How to calculate the number of stereoisomers of inositol homologs
Yajima, A.
Bull. Chem. Soc. Jpn. 87, 1260-1264 (2014).
doi:10.1246/bcsj.20140204

OEISに収録されました! リンク
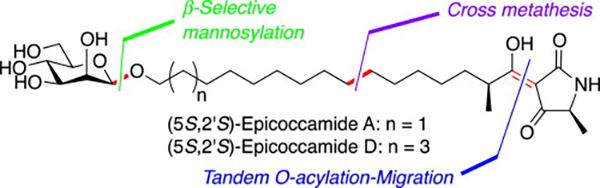
- Total synthesis of epicoccamides A and D via olefin cross-methathesis
Yajima, A.; Kawajiri, A.; Mori, A.; Katsuta, R.; Nukada, T.
Tetrahedron Lett. 55, 4350-4354 (2014).
doi:10.1016/j.tetlet.2014.06.040
- (Review article) Recent progress in the chemistry and chemical biology of microbial signaling molecules: quorum-sensing pheromones and microbial hormones
Yajima, A.
Tetrahedron Lett. 55, 2773-2780 (2014).
doi:10.1016/j.tetlet.2014.03.051

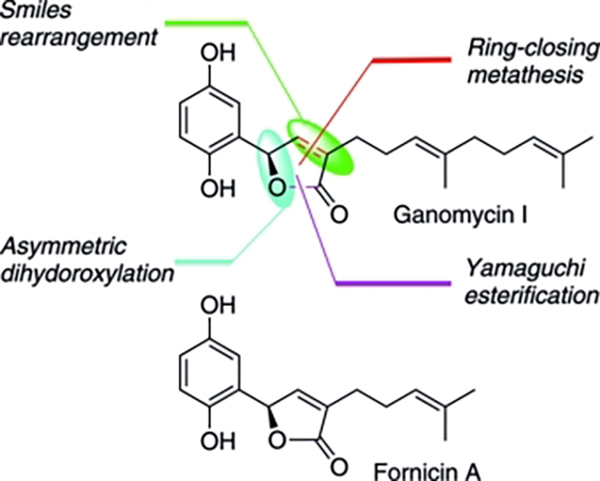
- Concise syntheses and biological activities of ganomycin I and fornicin A
Yajima, A.; Urao, S.; Katsuta, R.; Nukada, T.
Eur. J. Org. Chem. 2014, 731-738 (2014).
doi:10.1002/ejoc.201301269
2013
- Ubiquitin-specific peptidase 5, a target molecule of vialinin A, is a key molecule of TNF-a production in RBL-2H3 cells
Yoshioka, Y.; Ye, Y. Q.; Okada, K.; Taniguchi, K.; Yoshida, A.; Sugaya, K.; Onose, J.; Koshino, H.; Takahashi, S.; Yajima, A.; Yajima, S.; Abe, N.
PLoS ONE 8, e80931
doi:10.1371/journal.pone.0080931
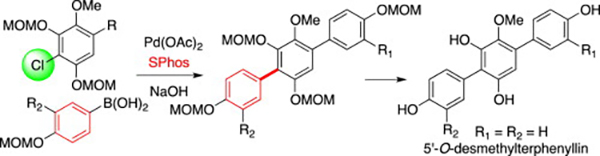
- Syntheses of 5′-O-desmethylterphenyllin and related p-terphenyls and their inhibitory activity of TNF-α release from RBL-2H3 cells
Yajima, A.; Urao, S.; Yoshioka, S.; Abe, N.; Katsuta, R.; Nukada, T.
Tetrahedron Lett. 54, 4986-4989 (2013).
doi:10.1016/j.tetlet.2013.07.062
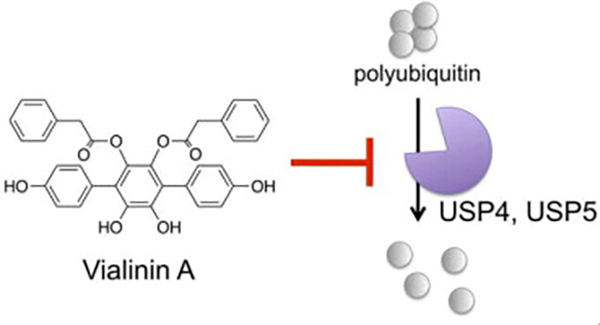
- Vialinin A is a ubiquitin-specific peptidase inhibitor
Okada, K.; Ye, Y. Q.; Taniguchi, K.; Yoshida, A.; Akiyama, T.; Yoshioka, Y.; Onose, J.; Koshino, H.; Takahashi, S.; Yajima, A.; Abe, N.; Yajima, S.
Bioorg. Med. Chem. Lett. 23, 4328-4331 (2013).
doi:10.1016/j.bmcl.2013.05.093
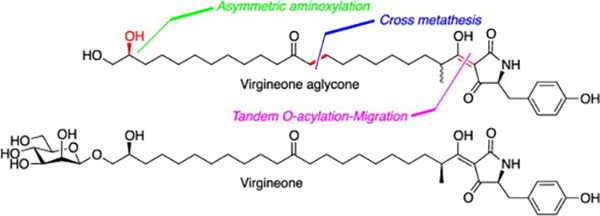
- Total synthesis of virgineone aglycone and stereochemical assignment of natural virgineone
Yajima, A.; Ida, C.; Taniguchi, K.; Murata, S.; Katsuta, R.; Nukada, T.
Tetrahedron Lett. 54, 2497-2501 (2013).
doi:10.1016/j.tetlet.2013.03.006
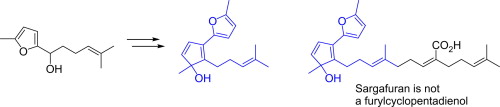
- Synthesis of the core framework of the proposed structure of sargafuran
Katsuta, R.; Aoki, K.; Yajima, A.; Nukada, T.
Tetrahedron Lett. 54,v 347-350 (2013).
doi:10.1016/j.tetlet.2012.11.052
2012
- Inhibitory effects of vialinin A and its analog on tumor necrosis factor-α release and production from RBL-2H3 cells
Onose, J.; Yoshioka, Y.; Ye, Q.; Sugaya, K.; Yajima, A.; Taniguchi, K.; Okada, K.; Yajima, S.; Takahashi, S.; Koshino, H.; Abe, N.
Cell. Immunol. 279, 140-144 (2012).
doi:10.1016/j.cellimm.2012.10.008
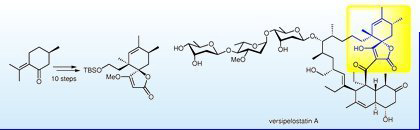
- Synthetic study of versipelostatin A: Synthesis of the spirotetronate unit starting from pulegone
Katsuta, R.; Arai, K.; Yajima, A.; Nukada, T.
Synlett 23, 397-400 (2012).
doi:10.1055/s-0031-1290204
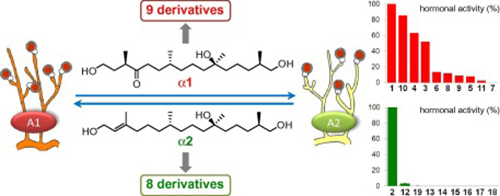
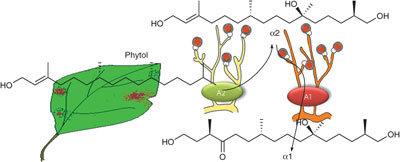
- Structure-activity relationship of a hormones, the mating factors of phytopathogen Phytophthora
Molli, S. D., Qi, J.; Yajima, A.; Shikai, K.; Imaoka, T.; Nukada, T.; Yabuta, G.; Ojika, M.
Bioorg. Med. Chem. 20, 681-686 (2012).
doi:10.1016/j.bmc.2011.12.015
- Synthesis of two osteoclast-forming suppressors, demethylincisterol A3 and chaxine A
Yajima, A.; Kagohara, Y.; Shikai, K.; Katsuta, R.; Nukada, T.
Tetrahedron 68, 1729-1735 (2012).
doi:10.1016/10.1016/j.tet.2011.12.057

2011
- Synthesis of the four stereoisomers of Phytophthora mating hormone a2 and a concise synthesis of mating hormone a1
Yajima, A.; Toda, K.; Molli, S. D.; Ojika, M.; Nukada, T.
Tetrahedron 67, 8887-8894 (2011).
doi:10.1016/10.1016/j.tet.2011.09.066
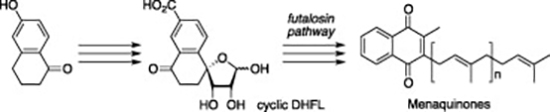
- Synthesis of (±)-cyclic dehypoxanthine futalosine, the biosynthetic intermediate in an alternative biosynthetic pathway for menaquinones
Yajima, A.; Kouno, S.; Dairi, T.; Mogi, M.; Katsuta, R.; Seto, H.; Nukada, T.
Tetrahedron Lett. 52, 4934-4937 (2011).
doi:10.1016/j.tetlet.2011.07.061
- The second Phytophthora mating hormone defines interspecies biosynthetic crosstalk
Ojika, M.; Molli, S. D.; Kanazawa, H.; Yajima, A.; Toda, K.; Nukada, T.; Mao, H.; Murata, R.; Asano, T.; Qi, J.; Sakagami, Y.
Nat. Chem. Biol. 7, 591-593 (2011).
doi:10.1038/nchembio.617
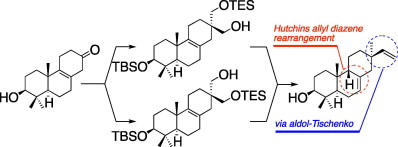
- Stereocontrolled total synthesis of (±)-3b-hydroxy-9b-pimara-7,15-diene, a putative biosynthetic intermediate of momilactones
Yajima, A.; Toda, K.; Okada, K.; Yamane, H.; Yamamoto, M.; Hasegawa, M.; Katsuta, R.; Nukada, T.
Tetrahedron Lett. 52, 3212-3215 (2011).
doi:10.1016/j.tetlet.2011.04.044
- (Review article) Approaches to dihydrooxazine ring systems and application in the synthesis of bioactive natural products
Gu, Z.; Saito, T.; Zakarian, A.
Chem. Heterocycl. Com. 48, 11-16 (2011).
doi:10.1007/s10593-012-0961-y
- (Review article) Synthesis of microbial signaling molecules and their stereochemistry-activity relationship
Yajima, A.
Biosci. Biotechnol. Biochem. 75, 1418-1429 (2011).
doi:10.1271/bbb.110283












































